Mechanism and Reactions of Dibutyl Sulfide
Ascent Fine Chemical
Mechanism and Reactions of Dibutyl Sulfide
Dibutyl sulfide (DBS) is an organosulfur compound with the chemical formula
C8H18S
It is a versatile chemical used in various applications, including as an agricultural intermediate, a sulfiding agent, a refinery catalyst, a lubricant additive, a gas odorant, and a processing aid in mining applications. The compound is also used as an internal standard in X-ray fluorescence spectrometry (XRF) measurements of sulfur in oils and other liquid hydrocarbon matrices.
- Oxidation Reactions
DBS can undergo oxidation reactions to form sulfoxides and sulfones, which are important intermediates in organic synthesis and industrial processes. The oxidation of sulfides to sulfoxides and sulfones can be achieved through various oxidants, including hydrogen peroxide and ozone.
- Oxidation with Hydrogen Peroxide
The oxidation of sulfides to sulfoxides using hydrogen peroxide is a well-documented reaction. Hydrogen peroxide is a popular oxidant due to its environmentally friendly nature, as its main by-product is water.[1]The oxidation typically proceeds through the formation of a peroxysulfurane intermediate, which then cleaves to form the sulfoxide and water. The reaction can be catalyzed by various substances, including metalloporphyrins and organocatalysts.[1, 2]
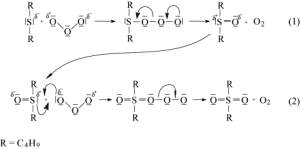
- Oxidation with Ozone
The oxidation of DBS with ozone has been studied, and a scheme of the reaction and generation of transitional products has been determined.[3]The main identified intermediate product was dibutyl sulfoxide (DBSO), and the main end product was dibutyl sulfone (DBSO2). The reaction proceeds through the electrophilic attack of ozone on the sulfur atom of DBS, leading to the formation of the sulfoxide. Further oxidation of the sulfoxide with ozone is more challenging due to the delocalized negative charge on the ozone molecule and the relatively small positive charge on the sulfur atom in the sulfoxide molecule. This results in a slower oxidation process, transforming the sulfoxide into sulfone.[3]
- Physical and Chemical Properties
DBS is a light yellow liquid with a distinct odor, often described as stench. It has a boiling point of 188-189 °C and a melting point of -76 to -80 °C. The vapor pressure of DBS is 5.17 mmHg at 37.7 °C, and it has a density of 0.838 g/mL at 25 °C. DBS is slightly miscible with water but miscible with olive oil and almond oil. The compound is considered combustible and can cause skin and eye irritation upon contact. It is also toxic if inhaled and may have effects on the central nervous system.
- Skolia, E., Gkizis, P. L., Nikitas, N. F., & Kokotos, C. G. (2022). Photochemical aerobic oxidation of sulfides to sulfoxides: the crucial role of wavelength irradiation. Green Chemistry, 24(10), 4108-4118., Photochemical aerobic oxidation of sulfides to sulfoxides: the crucial role of wavelength irradiation.(2022).
- Kupwade, R., A Concise Review on Synthesis of Sulfoxides and Sulfones with Special Reference to Oxidation of Sulfides.Journal of Chemical Reviews, 2019. 1.
- Popiel, S., et al., Effect of temperature and initial dibutyl sulfide concentration in chloroform on its oxidation rate by ozone.Journal of Hazardous Materials, 2008. 157(2): p. 319-327.

